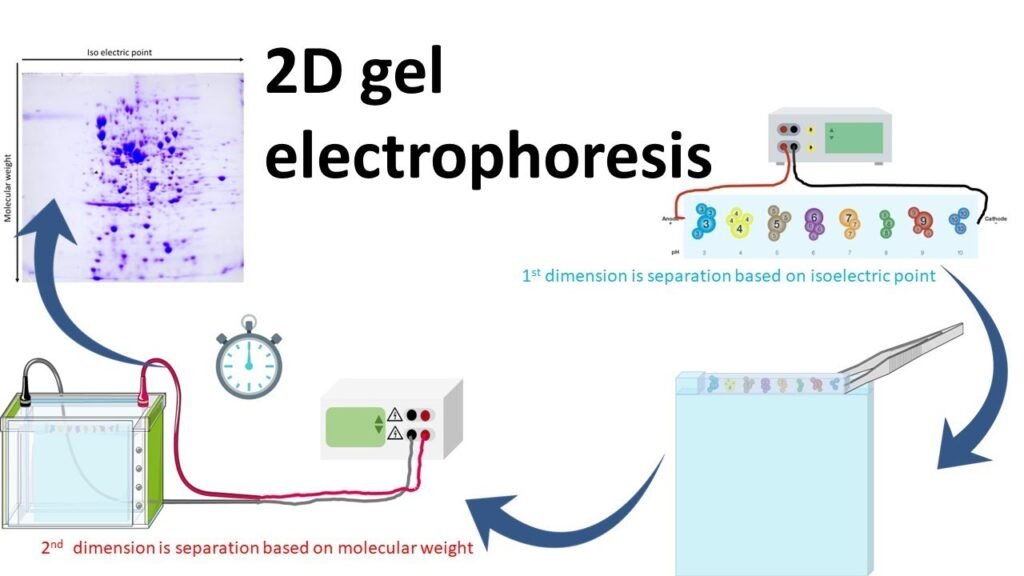
2D gel electrophoresis (2D GE) is an advanced laboratory technique used to separate and analyze complex mixtures of proteins. It is widely used in the field of proteomics to identify, quantify, and characterize proteins based on two distinct properties: isoelectric point (pI) and molecular weight. The ability of 2D gel electrophoresis to resolve thousands of proteins in a single experiment makes it invaluable for research in biology, biochemistry, and medicine.
This article explains how 2D gel electrophoresis functions, with a focus on the 2D protein gel method, and outlines the step-by-step process involved in the separation and analysis of proteins.
Understanding 2D Protein Gel Electrophoresis
The “2D” in 2D protein gel electrophoresis refers to two-dimensional separation. Proteins are first separated by their isoelectric point in one dimension (isoelectric focusing) and then by their molecular weight in the second dimension (SDS-PAGE). These two parameters provide a high-resolution map of protein mixtures, allowing researchers to analyze them in great detail.
The 2D protein gel process typically involves the following two key stages:
Isoelectric Focusing (IEF) – First Dimension Separation
The first dimension of 2D protein gel electrophoresis is isoelectric focusing, which separates proteins based on their isoelectric point (pI). The pI is the pH at which a protein carries no net charge, meaning the number of positive and negative charges in the protein balance out.
How It Works:
Preparation of the Gel Strip: Proteins are first applied to a gel strip containing an immobilized pH gradient (IPG). This strip has varying pH values from one end to the other, creating a gradient from acidic to basic conditions.
Electric Field Application: When an electric field is applied across the strip, proteins migrate through the gel based on their charge. Positively charged proteins move towards the cathode, while negatively charged proteins move towards the anode.
Isoelectric Point: As proteins move along the pH gradient, they stop moving when they reach the region where the pH matches their isoelectric point. At this point, the protein carries no net charge and remains stationary. The result is a separation of proteins based on their individual pI values.
This stage of 2D protein gel separation ensures that proteins with similar molecular weights but different charges are separated in the first dimension.
SDS-PAGE – Second Dimension Separation
Once the proteins are separated based on their isoelectric point, the second step of 2D protein gel electrophoresis is SDS-PAGE (Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis), which separates proteins based on their molecular weight.
How It Works:
Denaturation of Proteins: After isoelectric focusing, the proteins are treated with sodium dodecyl sulfate (SDS), a detergent that denatures the proteins and gives them a uniform negative charge. SDS binds to the proteins and eliminates differences in charge, so all proteins now move through the gel based only on their size (molecular weight).
Polyacrylamide Gel: The proteins are then loaded onto a polyacrylamide gel, where an electric field is applied. Because all proteins have the same negative charge due to the SDS treatment, they migrate towards the anode, with smaller proteins moving faster through the gel matrix than larger ones. This leads to a size-based separation of proteins.
Visualization and Analysis
After proteins are separated by 2D protein gel electrophoresis, they are visualized using staining techniques. Commonly used stains include Coomassie Brilliant Blue and silver staining, which allow researchers to detect individual protein spots on the gel.
Spot Identification: Each spot on the 2D protein gel represents a unique protein or protein isoform. By comparing the positions of spots across different samples or conditions, researchers can identify differences in protein expression or modifications.
Quantification: The intensity of each protein spot is proportional to the amount of protein present. This allows for relative quantification of protein abundance across samples.
For further analysis, protein spots of interest can be excised from the gel and subjected to techniques such as mass spectrometry (MS) for precise identification and characterization of the proteins.
Applications of 2D Protein Gel Electrophoresis
2D protein gel electrophoresis is a versatile technique that finds application in a wide range of biological and biomedical research areas. Some key applications include:
Proteomics Research
In proteomics, researchers study the entire set of proteins expressed by a cell or organism. 2D protein gel electrophoresis is a foundational tool for identifying and cataloging proteins, analyzing protein expression patterns, and studying how proteins respond to changes in the environment or disease conditions.
Post-Translational Modification (PTM) Analysis
Proteins undergo various post-translational modifications, such as phosphorylation, glycosylation, and ubiquitination, which regulate their function. 2D protein gel can effectively separate modified and unmodified versions of the same protein, enabling the study of PTMs and their impact on biological processes.
Biomarker Discovery
By comparing the proteomes of healthy and diseased cells or tissues, 2D protein gel electrophoresis can help identify protein biomarkers associated with specific diseases. These biomarkers can be used for early diagnosis, treatment monitoring, and drug development.
Drug Development and Toxicology Studies
2D protein gel is used in drug development to study the effects of new drugs on protein expression and modification. It also helps identify off-target effects and potential toxicity by analyzing how protein profiles change in response to drug treatment.
Benefits of 2D Protein Gel Electrophoresis
The use of 2D protein gel electrophoresis offers several advantages for protein analysis:
High Resolution: The technique separates proteins based on two distinct properties (pI and molecular weight), resulting in high-resolution separation and the ability to analyze complex protein mixtures.
Comprehensive Proteome Coverage: Researchers can resolve and analyze thousands of proteins from a single sample, making it ideal for large-scale proteomic studies.
Quantitative and Qualitative Analysis: 2D protein gel allows for both quantification of protein expression and analysis of protein modifications, making it a valuable tool for understanding protein function.
Cost-Effective: Compared to more advanced proteomic techniques, 2D protein gel electrophoresis is relatively affordable and does not require expensive instrumentation.
Conclusion
2D protein gel electrophoresis is a powerful and reliable method for separating and analyzing proteins based on their isoelectric point and molecular weight. Its high resolution, ability to analyze complex protein mixtures, and applications in proteomics, biomarker discovery, and drug development make it an indispensable tool in molecular biology and biochemistry. Researchers who aim to study proteins in-depth will find 2D protein gel an effective and versatile technique to explore protein expression, modification, and interaction.
Whether you’re conducting basic research or applied studies in medicine or biotechnology, 2D protein gel electrophoresis offers a detailed and accurate approach to understanding proteins and their roles in biological systems.Bottom of Form